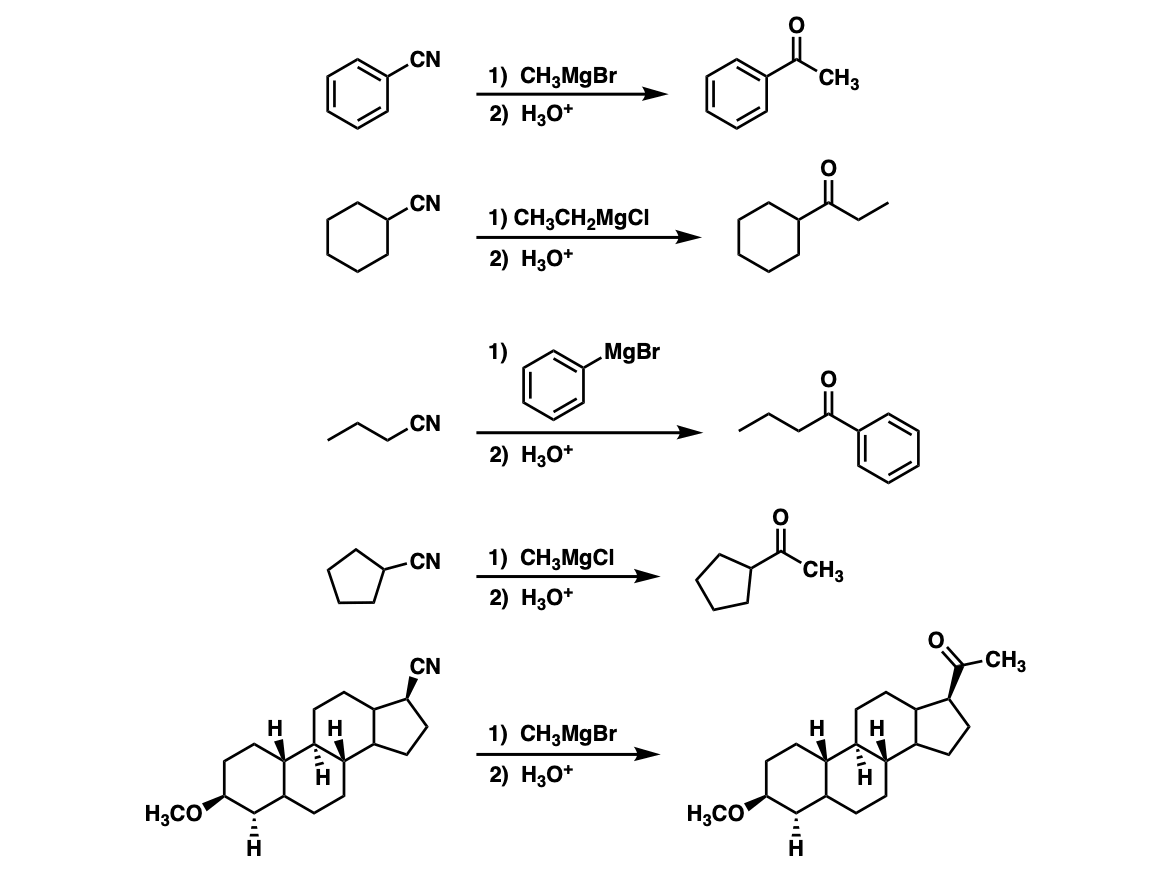
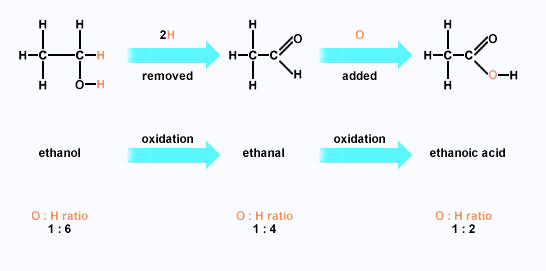
The proposed mechanism for the reaction of methyl vinyl ketone with Cl... | Download Scientific Diagram

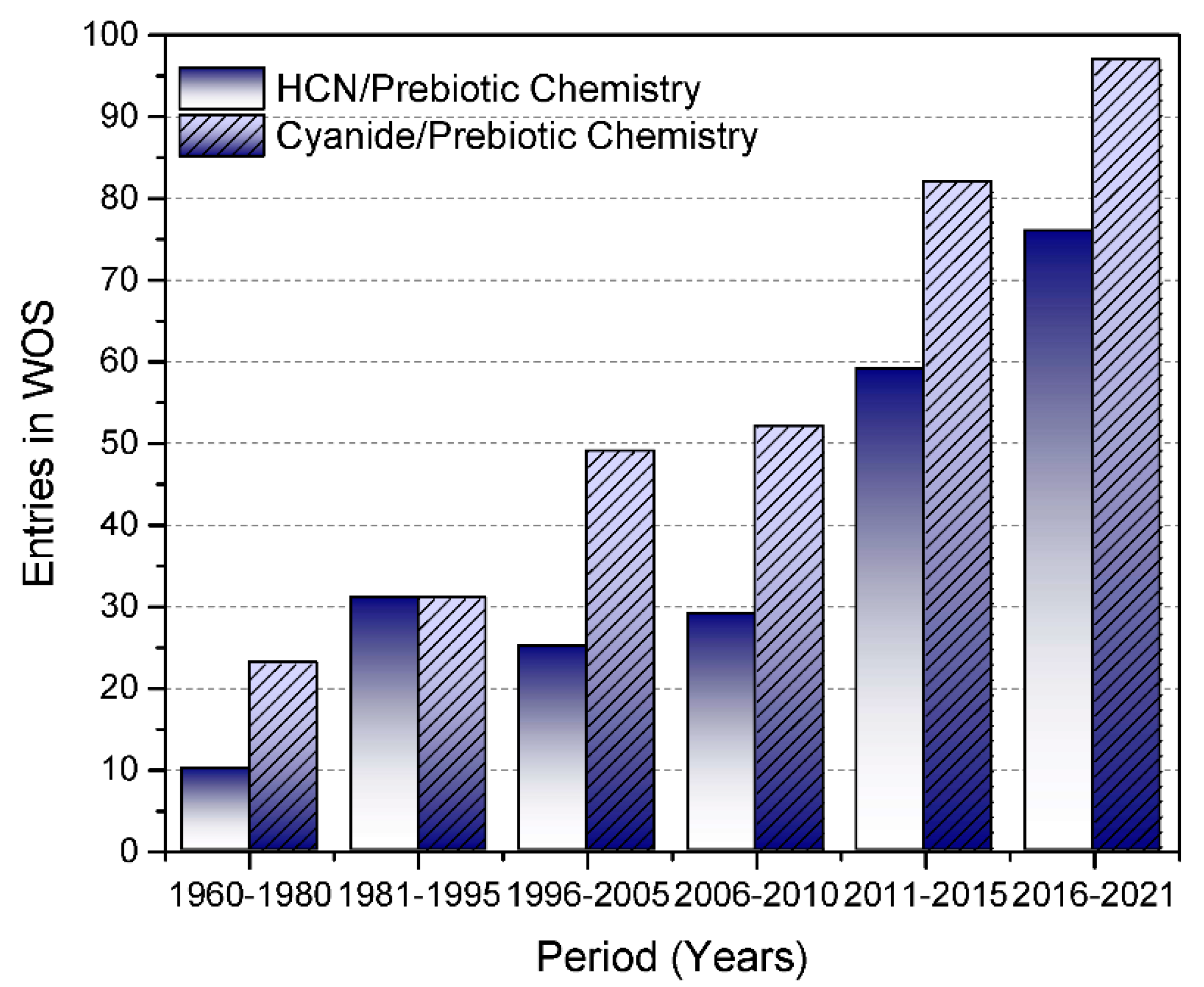
HCN on Tap: On-Demand Continuous Production of Anhydrous HCN for Organic Synthesis | Organic Letters

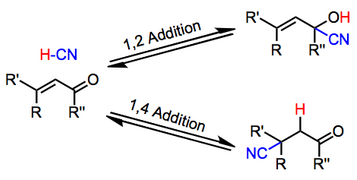
Asymmetric Multicomponent Domino Reactions and Highly Enantioselective Conjugated Addition of Thiols to α,β-Unsaturated Aldehydes | Journal of the American Chemical Society

Acetone Cyanohydrin as a Source of HCN in the Cu-Catalyzed Hydrocyanation of α-Aryl Diazoacetates | The Journal of Organic Chemistry

The proposed mechanism for the reaction of methyl vinyl ketone with Cl... | Download Scientific Diagram

HCN on Tap: On-Demand Continuous Production of Anhydrous HCN for Organic Synthesis | Organic Letters
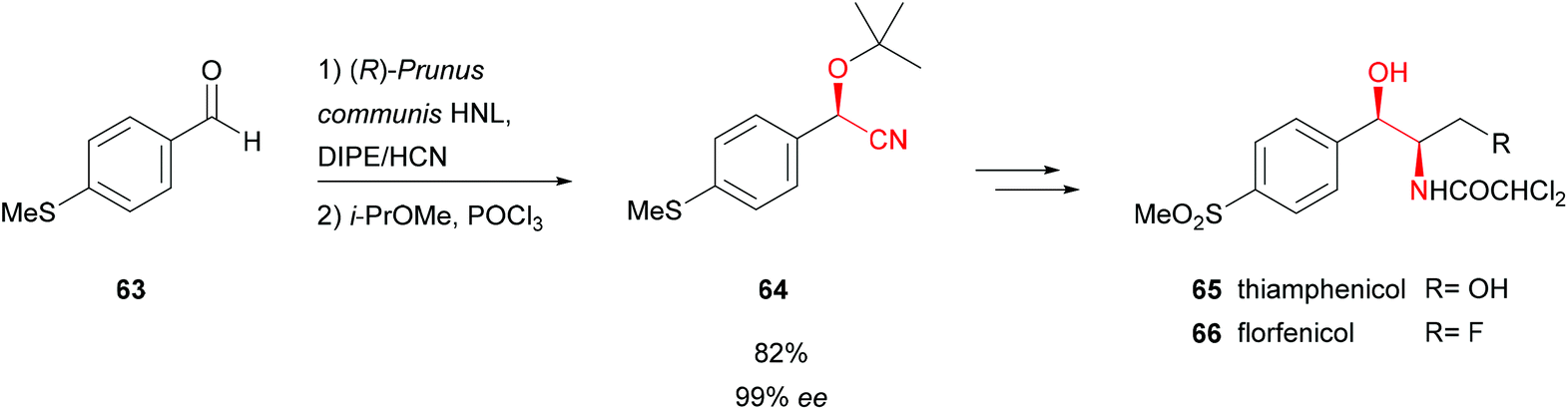
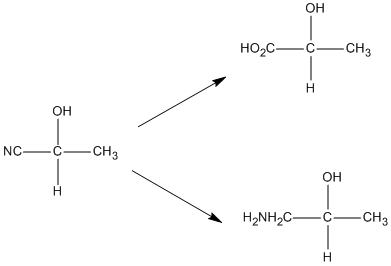
Enantioselective synthesis of cyanohydrins catalysed by hydroxynitrile lyases – a review - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C6OB00934D
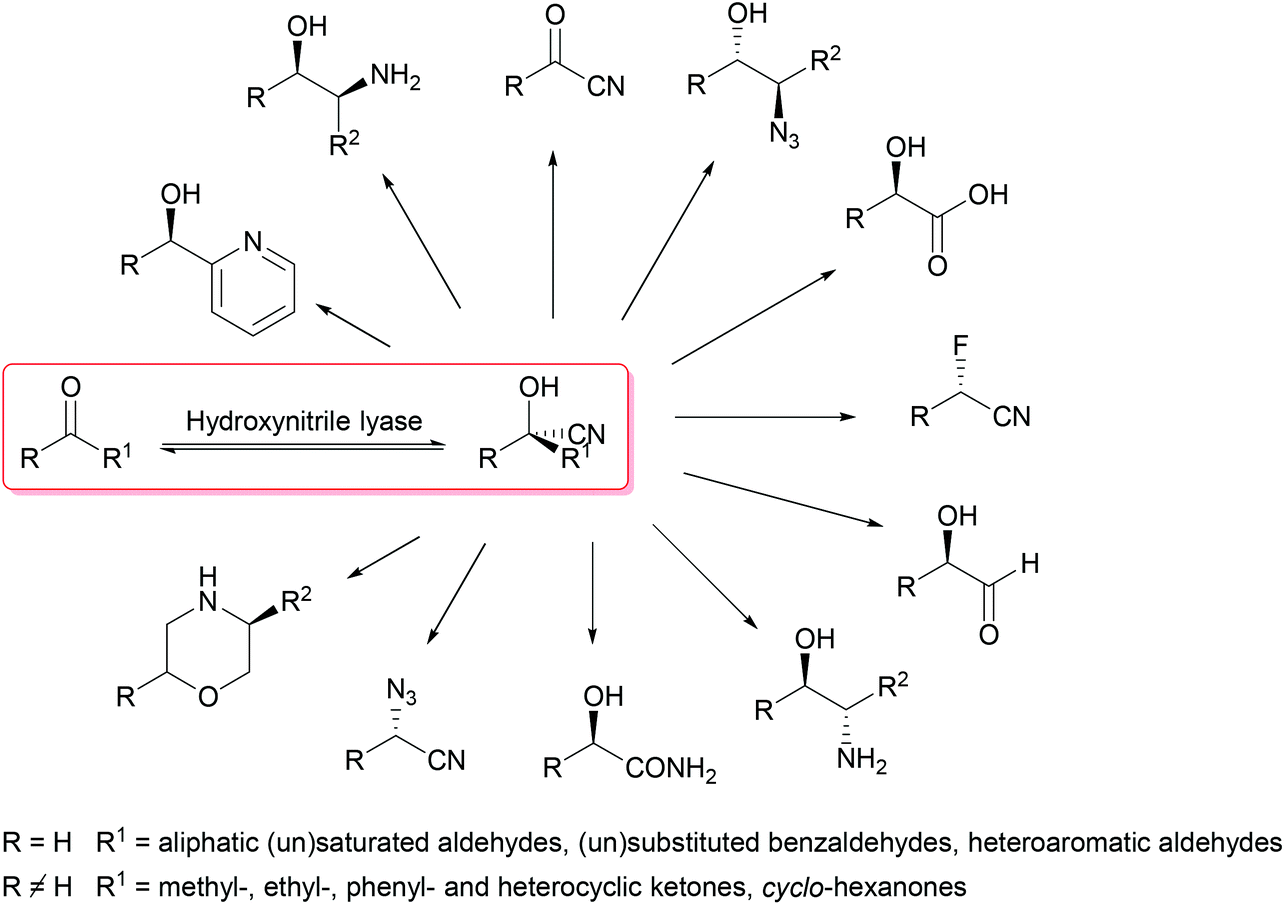
Enantioselective synthesis of cyanohydrins catalysed by hydroxynitrile lyases – a review - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C6OB00934D